Featured Scientist
Wei-Ning Lin
Wei-Ning Lin
Graduate Institution of Biomedical and Pharmaceutical Science,
Fu Jen Catholic University, New Taipei City, Taiwan
Article published in
"Journal of Clinical Medicine" 2018, 7(9), 266
Translational Medicine in Pulmonary-Renal Crosstalk: Therapeutic Targeting of p-Cresyl Sulfate Triggered Nonspecific ROS and Chemoattractants in Dyspneic Patients with Uremic Lung Injury
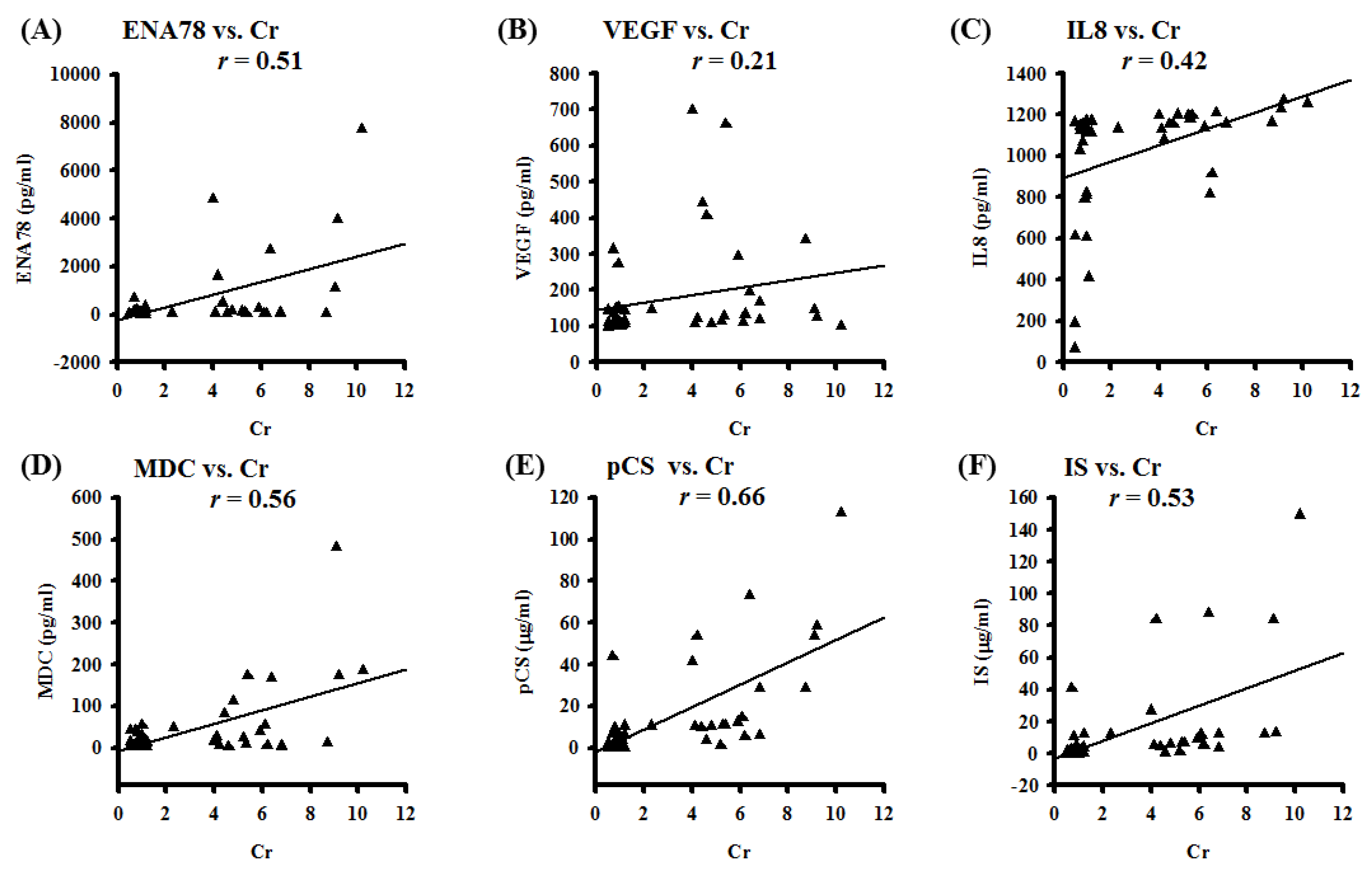
Molecular mechanisms and pathological features of p-Cresyl sulfate (PCS)-induced uremic lung injury (ULI) in chronic kidney disease (CKD) remain unclear. We analyzed pleural effusions (PE) from CKD and non-CKD patients for uremic toxins, reactive oxygen species (ROS), and chemotactic cytokines. Correlations between PE biomarkers and serum creatinine were also studied. Cell viability and inflammatory signaling pathways were investigated in PCS-treated human alveolar cell model. To mimic human diseases, CKD-ULI mouse model was developed with quantitative comparison of immunostaining and morphometric approach. PE from CKD patients enhance expressions of uremic toxins, hydroxyl radicals, and IL-5/IL-6/IL-8/IL-10/IL-13/ENA-78/GRO α/MDC/thrombopoietin/VEGF. PE concentrations of ENA-78/VEGF/IL-8/MDC/PCS/indoxyl sulphate correlate with serum creatinine concentrations. In vitro, PCS promotes alveolar cell death, cPLA2/COX-2/aquaporin-4 expression, and NADPH oxidase/mitochondria activation-related ROS. Intracellular ROS is abrogated by non-specific ROS scavenger N-acetyl cysteine (NAC), inhibitors of NADPH oxidase and mitochondria-targeted superoxide scavenger. However, only NAC protects against PCS-induced cell death. In vivo, expressions of cPLA2/COX2/8-OHdG, resident alveolar macrophages, recruited leukocytes, alveolar space, interstitial edema and capillary leakage increase in lung tissues of CKD-ULI mice, and NAC pretreatment ameliorates alveolar–capillary injury. PCS causes alveolar–capillary injury through triggering intracellular ROS, downstream prostaglandin pathways, cell death, and activating leukocytes to release multiplex chemoattractants and extracellular ROS. Thus PCS and nonspecific ROS serve as potential therapeutic targets. [Full article]