Featured Scientist

Author published in "J. Hazard. Mater." affiliate to
Bing-Huei Chen
Department of Food Science,
Fu Jen Catholic University, New Taipei City, Taiwan
Article published in
"Journal of Hazardous Materials" Volume 415, 5 August 2021, 125701
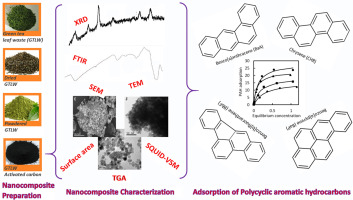
Removal of polycyclic aromatic hydrocarbons from water by magnetic activated carbon nanocomposite from green tea waste
This study aims to synthesize a magnetic activated carbon nanocomposite from green tea leaf waste (MNPs-GTAC) for evaluation of adsorption efficiency of 4 priority polycyclic aromatic hydrocarbons (PAHs). MNPs-GTAC contained spherically-shaped MNPs with cubic spinel structure, surface area at 118.8 m2/g, particle size at 8.6 nm and saturation magnetization at 34.2 emu/g. PAH adsorption reached a plateau at an MNPs-GTAC dose of 50 or 60 mg/L, pH of 2–4 and ionic strength of 0.1–10%, with PAH reduction in the presence of humic acid being compensated by addition of 0.1% sodium chloride. Kinetics was rapid attaining 80% removal within 5 min and the pseudo-second-order rate decreased in this order: Benzo[a]anthracene>Chrysene>Benzo[b]fluoranthene>Benzo[a]pyrene. Isotherm modeling revealed a Langmuir type-2 shape with the maximum adsorption capacity being 28.08, 22.75, 19.14 and 15.86 mg/g for Benzo[b]fluoranthene, Benzo[a]pyrene, Chrysene and Benzo[a]anthracene, respectively. Temperature study showed the PAH adsorption to be an endothermic and spontaneous process with increased randomness at solid-solution interface. Acetonitrile could completely recover the adsorbed PAH and MNPs-GTAC was successfully recycled 5 times with a minimum loss. Application to mineral water showed 86–98% and 72–89% removal for PAHs spiked respectively at 0.1 and 1 mg/L, while a complete removal was attained in tap and river waters.
Keywords: Magnetic nanoparticlesGreen tea waste activated carbonMagnetic carbon nanocompositePolycyclic aromatic hydrocarbonsAdsorption mechanism
57 views