Featured Scientist

Author published in"Nano Letters"affiliate to
College of Science and Engineering
Hsiao-Ching Yang
Department of Chemistry,
Fu Jen Catholic University, New Taipei City, Taiwan
Article published in
"Nano Letters" 2020, 20, 9, 6630–6635
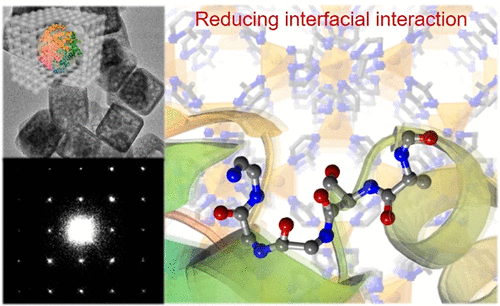
Probing Interactions between Metal–Organic Frameworks and Freestanding Enzymes in a Hollow Structure
It has been reported that the biological functions of enzymes could be altered when they are encapsulated in metal–organic frameworks (MOFs) due to the interactions between them. Herein, we probed the interactions of catalase in solid and hollow ZIF-8 microcrystals. The solid sample with confined catalase is prepared through a reported method, and the hollow sample is generated by hollowing the MOF crystals, sealing freestanding enzymes in the central cavities of hollow ZIF-8. During the hollowing process, the samples were monitored by small-angle X-ray scattering (SAXS) spectroscopy, electron microscopy, powder X-ray diffraction (PXRD), and nitrogen sorption. The interfacial interactions of the two samples were studied by infrared (IR) and fluorescence spectroscopy. IR study shows that freestanding catalase has less chemical interaction with ZIF-8 than confined catalase, and a fluorescence study indicates that the freestanding catalase has lower structural confinement. We have then carried out the hydrogen peroxide degradation activities of catalase at different stages and revealed that the freestanding catalase in hollow ZIF-8 has higher activity.[Full article]
KEYWORDS:metal−organic frameworks enzyme immobilization hollow MOF interface small-angle X-ray scattering
81 views