Featured Scientist

Yi-Ju Tsai, Ph.D.
Professor
Graduate Institute of Biomedical and Pharmaceutical Science
Pain signaling and sensory plasticity laboratory
Chronic pain is a major health problem worldwide; however, current managements for chronic pain are unsatisfactory. The major goals of our lab are to identify novel molecular and cellular mechanisms that underlie the genesis of chronic pain. Recently, we also began to explore the mechanisms underlying the resolution of acute pain, and these mechanisms are involved in the evolution of pain from acute phase to chronic stage. We employ a multidisciplinary approach including in vitro and in vivo electrophysiology, neuronal and glial cell biology, transgenic mice, and behavior testing. We believe that better understanding the mechanisms of pain induction and resolution will lead to development of novel therapeutic strategies for chronic pain.
Pathological pain pathway in upper limbs
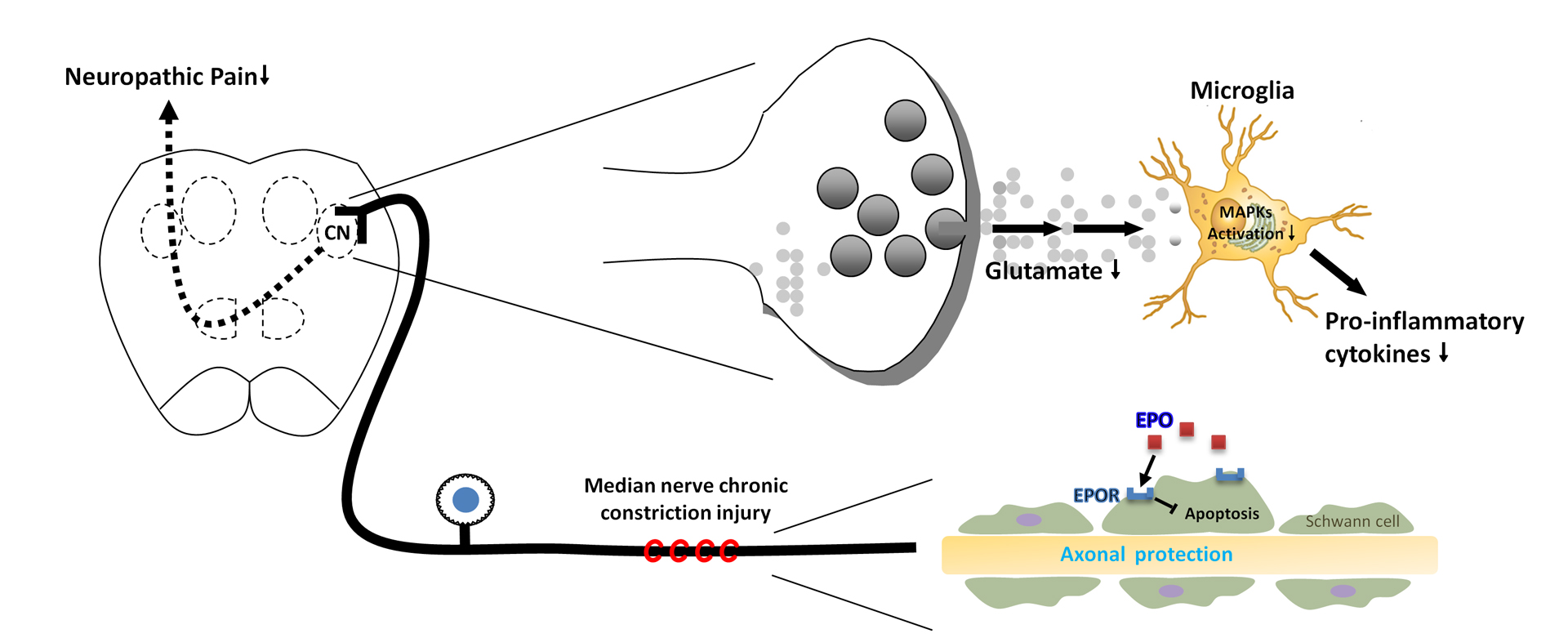
Based on previous studies, sensory signals, i.e., proprioception, vibration, touch and two-point discrimination, from upper limbs convey to the cuneate nucleus of the brainstem via Aα/β nerve fibers. The signals then project to the contralateral thalamus and subsequently ascend to the primary sensory cortex. The prior understanding is that this signaling pathway is not involved in pain sensation. Surprisingly, our lab shows that neurons and glial cells in the cuneate nucleus are activated following peripheral nerve injury of the upper limbs, and pain-associated substances, such as CGRP, GAL, NPY, SP, NOS and SOM, are identified in the above pathway. Moreover, injection of pain medications into the cuneate nucleus can achieve pain amelioration. Our findings revolutionize the conventional thinking about pain signal transmission and have been put in the neuroanatomy textbook.
The median nerve is the most commonly injured one amongst nerves of the upper limbs. However, there is paucity of researches about median nerve injury. In this regard, our lab successfully establishes a median nerve injury model of neuropathic pain to comprehensively investigate the signaling pathway of pathological pain in upper limbs.
Pathogenesis of pain via neural-glial interactions
We are interested in the following questions: (1) how neural signals, including electrical activity and release of proteases, in primary sensory neurons lead to the activation of glial cells, i.e., microglia and astrocytes, in the cuneate nucleus after median nerve injury, and (2) how glia mediators (cytokines and chemokines) modulate synaptic transmission in the cuneate nucleus. We demonstrate that distinct activation patterns of MAPK signaling pathways (ERK, p38, and JNK) in microglia and astrocytes in the cuneate nucleus are involved in and crucial for the development of neuropathic pain. We also show that proinflammatory cytokines, TNF-α and IL-1β, and chemokine, MCP-1, can modulate synaptic transmission by enhancing excitatory synaptic transmission and suppressing inhibitory synaptic transmission. These findings make significant progress towards a better understanding of the mechanisms of pathological pain.
Timing of therapeutic intervention and medications in pathological pain
We investigate the effects of therapeutic hypothermia and medications, i.e., lidocaine and melatonin, on nerve injury-induced neuropathic pain, and explore the optimal timing of those interventions. We demonstrate that administration of therapeutics prior to activation of glial cells achieves the best outcomes in alleviating pathological pain. The therapeutic interventions exert their effects via blocking sodium channels, attenuating ectopic discharge, resolving synaptic plasticity, and inhibiting inflammation and glial activation. Further, clinical observations indicate that disturbed sleep is not uncommon in patients with chronic pain disorders and inadequate sleep increases pain susceptibility. In this regard, we find that sleep deprivation increases vulnerability to nerve injury-induced neuropathic pain through the reduction of circulating melatonin, and restoration of melatonin circadian variation via exogenous supplementation in case of sleep disturbance improves pathological pain. The findings are practically useful and highlight the importance of tackling sleep disorders in patients suffering from pain in order to achieve optimal pain control.
82 views