Featured Scientist

Rwei-Fen Syu Huang, Ph.D.
Distinguished Professor
Department of Nutritional Science
Outstanding Research Award,Fu-Jen University
輔仁大學 優等學術研究獎
Excellent Research Award,Nutrition Society of Taiwan
台灣營養學會 學術研究傑出獎
Young Investigator Outstanding Research Award,
Nutrition Society of Taiwan
台灣營養學會 年輕優秀學者研究獎
University Outstanding Research Award,
Minister of Science and Technology, Taiwan.
台灣科技部補助大專校院特殊優秀人才獎勵
University of California at San Francisco, Visiting Scholar
加州大學舊金山分校 訪問學者
University of California at Berkeley, Visiting Scholar
加州大學柏克萊分校 訪問學者
Cornell University, Visiting Scholar
康乃爾大學 訪問學者
Editor-in-Chief, Nutritional Science Journal published by Nutrition Society of Taiwan
台灣營養學會期刊 總編輯
Guest Associate Editor, Mediator of Inflammation (SCI Journal)
客座副總編輯
Folate Nutrition in Metastatic Cancer Prevention and Prognosis
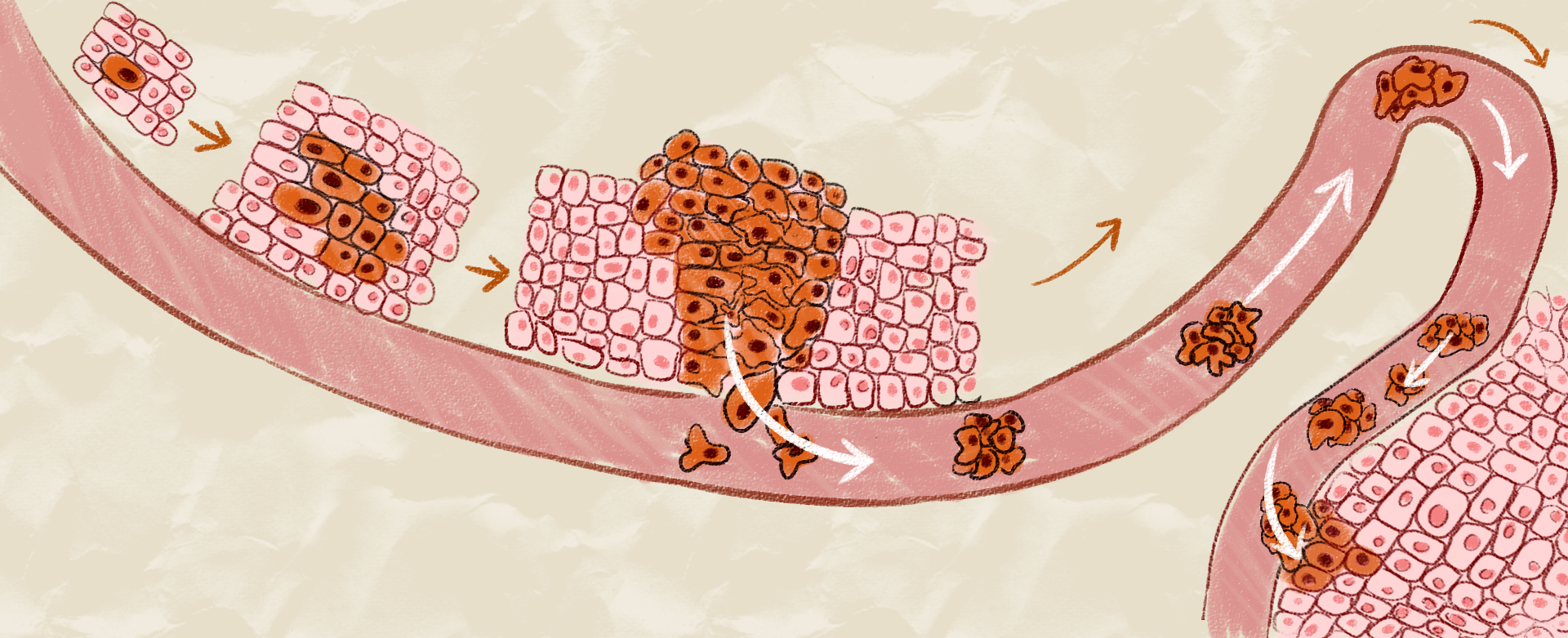
Lack of folate causes metabolic and oxidative stress in host cells, and this low folate-associated metabolic stress (LFMS) spurs carcinogenesis. Few studies have shown how LFMS induces metastasis potential of colon cancers. The Professor Rwei-Fen S. Huang’s research team tested 99 paired colonic tumours and non-cancer tissue samples to uncover such colon metastasis-promoting effects. The RFS Huang’s team observed that higher stage and more invasive tumors had depleted folate status associated with hypomethylation of Shh promoter region, the stemness marker, to activate the Shh signaling pathway, which boosted colonic cancer cells’ ability to invade and spread. The RFS Huang’s study highlights cancer stem cell epigenetic targets specific to LFMS-predisposed invasive colon cancers in optimizing folate co-chemotherapy to minimize cancer metastasis potential of patients with colonic cancers (International Journal of Cancer: 141, 2537-2550, 2017).
Professor RFS Huang’s research team continues to explore effects of nutritional low-folate stress on lung cancer metastasis, a cancer stem cell disease and the leading cause of worldwide cancer death. Cultivation of human non-small-cell lung cancers in an LF microenvironment enhanced metabostemness phenotype, reprogrammed Warburg bioenergetics to aerobic glycolysis, and promoted cancer invasion. Intrapleural injection of LF-induced lung oncospheres into the LF recipient mice, but not the control mice, caused metastasis lung tumours. The authors unveiled that LF metabolic stress reprograms metabostemness signatures through activated mTOR metabolic stress signaling to promote in vitro and in vivo metastasis potentials of human lung cancers (Journal of Nutritional Biochemistry. 53, 28-38, 2018).
113 views