Featured Scientist

Wen-Bin Wu, Ph.D.
Distinguished Professor
Exploring the role of neovascularization in folliculogenesis and oocyte maturation
Chronic inflammatory diseases are the most significant cause of death in the world. The prevalence of diseases associated with chronic inflammation is anticipated to increase persistently for the next 30 years in the world. For example, in 2017 cardiovascular diseases (CVDs) accounts for 1 out of every three deaths or approximately 800,000 deaths in the United States and three of five people die due to chronic inflammatory diseases.
“Chronic inflammation” refers to a prolonged inflammatory response that persists for months or years, generally involving a progressive change in the type of cells present at the site of inflammation and characterized by the simultaneous destruction and repair of the tissue (tissue remodeling). The immune system prompts white blood cells, generally macrophages and lymphocytes, to attack nearby healthy tissues, although in some cases, low-level inflammation becomes activated even when there is no apparent injury or disease. Some specific chronic inflammation-mediated diseases are CVDs, diabetes, allergies, rheumatoid arthritis, cancer, and etc.
Our laboratory is currently exploring the pathogenesis/pathophysiology that chronic inflammation plays in these disease conditions, and also investigating and developing agents which can protect from and treat these diseases using methodologies and approaches of cell and molecular biology and biochemical pharmacology. The following are our main focuses.
Studying the pathogenesis/pathophysiology of chronic inflammatory diseases and developing new health-care agents
An understanding of how chronic inflammation influences disease development and progression can lead to better treatment and prevention. Therefore, one of our focuses is to study the pathogenesis/pathophysiology of CVDs (especially atherosclerosis), diabetes with metabolic syndromes, and CRS. Our main approaches include analyzing human tissue samples, establishing in-vivo animal models mimicking disease status and in-vitro cell models. Currently we have established an apoE knockout atherosclerosis murine model, a carotid artery balloon-injury rat model, and diabetes with hyperlipidemia rat model. The animal model for CRS is under construction. In addition, in-vitro cell models established are vascular injury (inflammation) model, cell migration, and leukocyte-endothelial cell interaction model using primary vascular endothelial cells and smooth muscle cells (SMCs) from human umbilical cord, aortic SMCs from rat aorta, platelets from human whole blood, primary macrophages from peritoneal lavage of mice, and nasal mucosa-derived fibroblasts from CRS patient without nasal polyp. The nasal mucosa-derived epithelial cells are being setup in order to explore the pathophysiology of CRS with and without nasal polyp.
More precisely, our researches are focusing the effects of producing inflammatory cytokines, growth factors, enzymes and novel proteins/factors on the progression of these chronic diseases using human and established animal tissue samples and the characterized cells, exploring their roles during tissue damage and secondary repair (tissue remodeling). Our specialized techniques include cellular signaling, immunocyto (histo) chemistry, flowcyotmetry, ELISA, immunofluorescence, and molecular biology.
To overcome the chronic inflammatory diseases, carotenoids and nuclear receptor (NR) agonists can be as main sources for developing health-care products/drugs due to these substances generally with anti-inflammatory and antioxidant activity through affecting nuclear receptors (NRs). Carotenoids are colorful lipid-soluble pigments, which are divided into carotenes, xanthophylls and lycopene. Only about 40 carotenoids are present in a typical human diet and about 20 carotenoids have been identified in human blood and tissues. There are several NRs, including retinoic acid receptor (RARs), retinoid receptor (RXRs), peroxisome-proliferator activating receptor (PPAR), liver X receptor (LXR), farnesoid X receptor (FXR), and etc. An antidiabetic drug, pioglitazone (ACTOS®) in the thiazolidinedione class, works as an insulin sensitizer by binding to the PPAR in fat cells and making the cells more responsive to insulin. Therefore, we are interested in this area to develop derivatives of carotenoids and NR agonists to prevent and treat CVDs, metabolic syndromes and chronic rhinosinusitis (CRS).
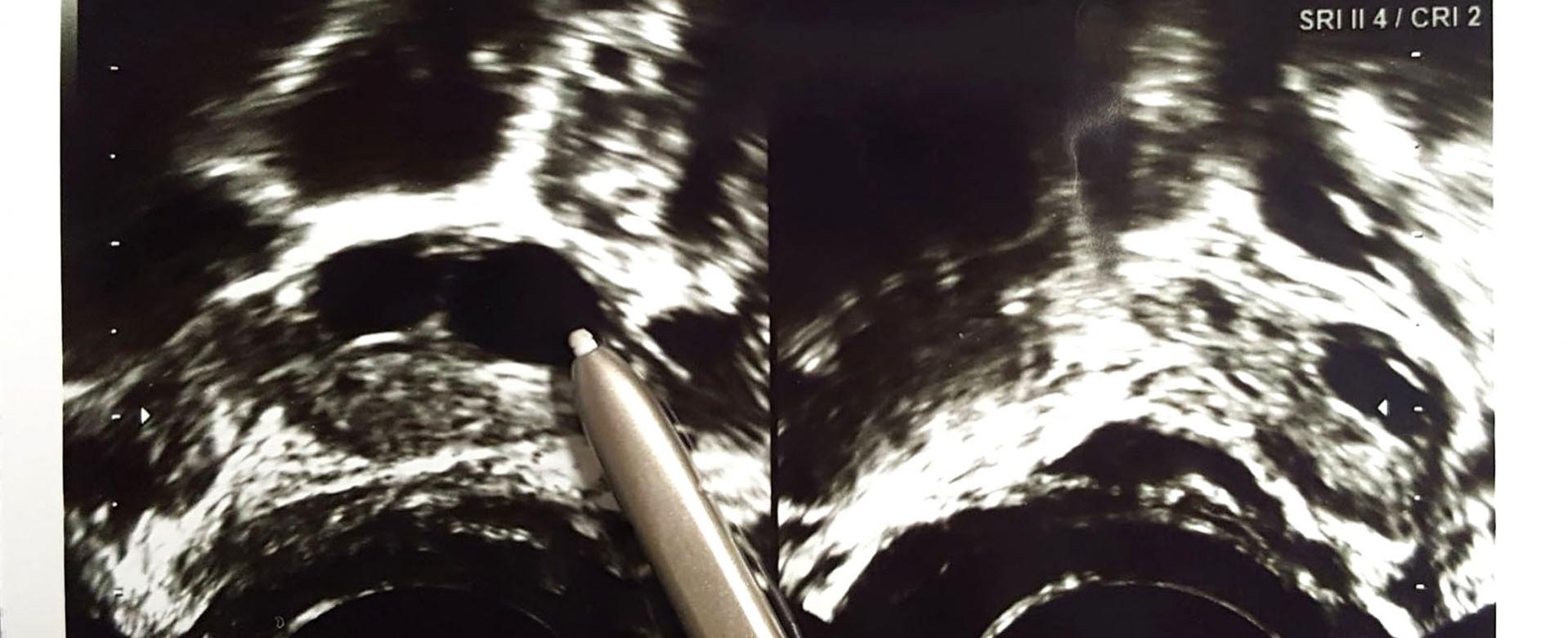
Exploring the role of neovascularization in folliculogenesis and oocyte maturation
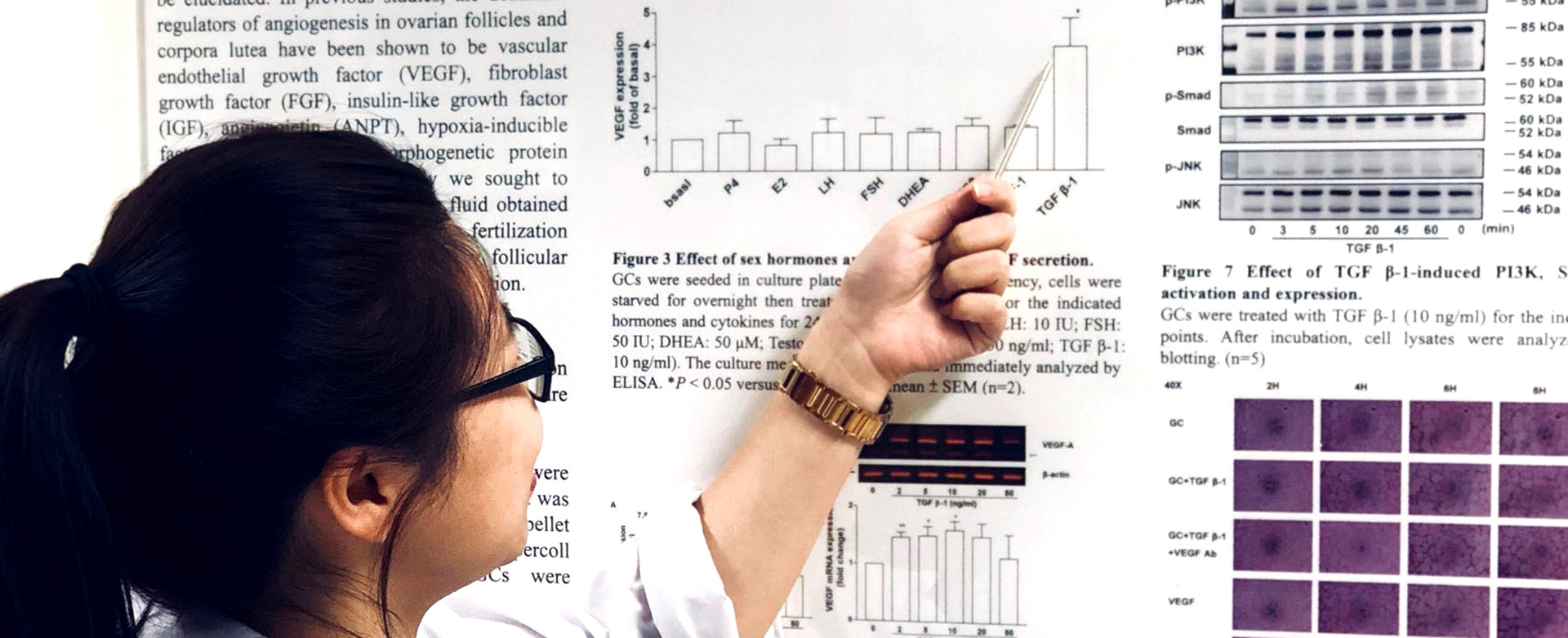
Another research focus is to search the novel key protein/factor (biomarker) responsible for neovascularization in folliculogenesis and develop a clinically available ELISA kit to predict oocyte quality. This is mainly based on the knowledge that healthy follicles are highly vascularized whereas those undergoing atresia have poor vascularity, suggesting a relationship between follicular vascularization and follicular function. A follicle structurally consists of oocyte in follicular fluid, which is surrounded by granulosa cells /cumulus cells, and theca cells. Interestingly, analysis of the components in follicular fluid reveals most of them are extracellular origin. Since neovascularization supports ovarian folliculogenesis, we hypothesize that the expression levels of cytokines and neovascularization-related factors in the human follicular fluid and follicular cells correlates with folliculogenesis and oocyte quality and maturation, consequently affecting the success rate of fertilization.
67 views