Confinements of silver nanoparticles in polystyrenes through molecular entanglements and their application for catalytic reduction of 4-nitrophenol
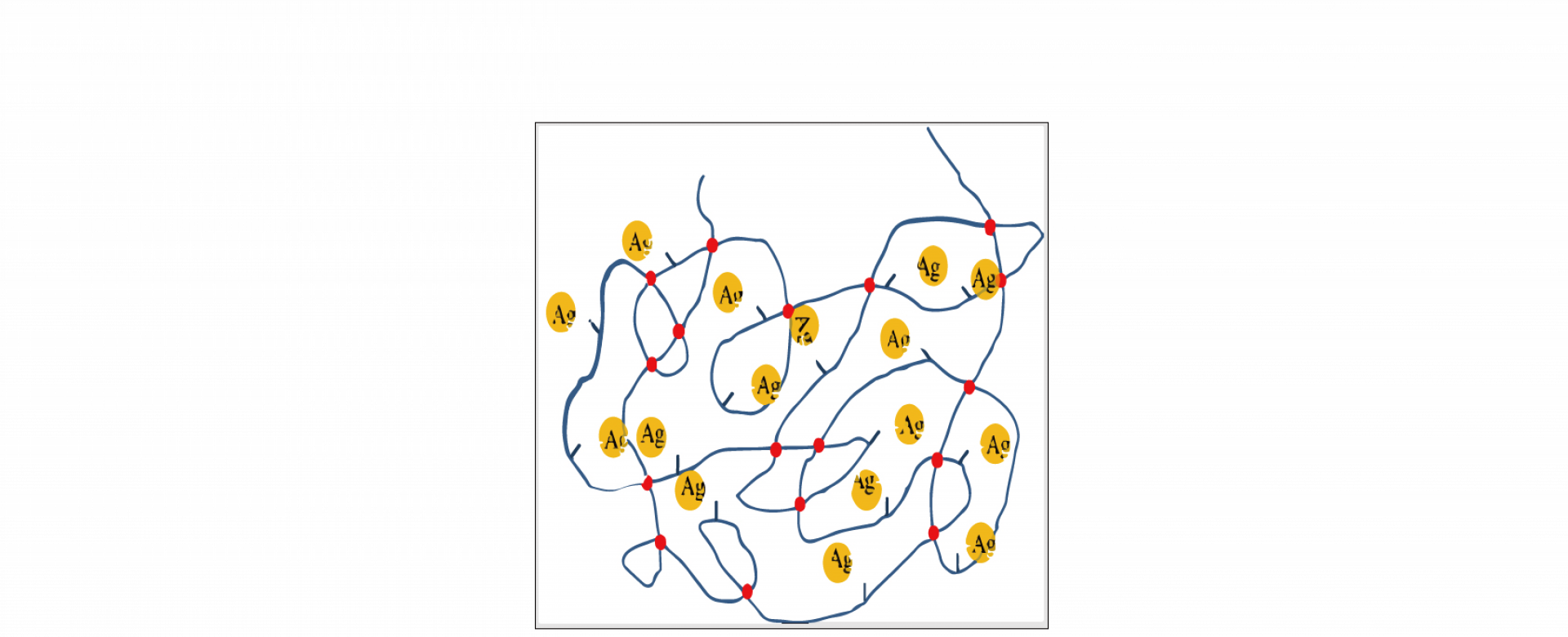
Nanoparticles have been widely studied for the catalytic application because of the increasing surface area from bulk materials to nanoparticles. It greatly increases the collision frequency between reactant and catalyst, leading to higher reaction rate. Although nanoparticles have advantages over bulk materials, aggregation of the nanoparticles at higher concentration (or drying process) has significantly diminished their intrinsic properties, especially, effective surface area. In other words, catalytic efficiency decreases dramatically because of decreasing effective surface area caused by the aggregation of nanoparticles. To minimize the aggregation behavior of nanoparticles, researchers have conducted different approaches to stabilize the nanoparticles. In this work, a soluble nano-silver containing polystyrene (PS-AgNPs) was synthesized to study how the molecular entanglements affected the aggregation of AgNPs. AgNPs were bonded to the side-chain of polystyrene so that mobilization of AgNPs was restricted by the entanglements of polystyrene. Catalytic reduction reaction of 4-nitrophenol using sodium borohydride (NaBH4) and different PS-AgNPs in THF/H2O mixed solvent system was selected to evaluate the catalytic capability of PS-AgNPs with different Ag concentrations. Experimental results showed that PS-AgNPs outperformed many other AgNPs on the catalytic efficiency of 4-nitrophenol. Additionally, PS-AgNPs demonstrated the very wide linear concentration range which cannot be achieved by traditional AgNPs.
To resolve the aggregation issue of AgNPs, students in Prof. Ping-Tsung Huang’s lab, Yu-Ning Chen and Shun-Huei Wu, synthesized three types of nanosilver containing polystyrenes. Thiol functional group was incorporated into the side-chain of polystyrene and silver was bonded to the thiol functional group. By controlling the amount of Ag in polystyrene, PS-AgNPs became soluble in solvent such as THF, dioxane, and toluene. The PS-AgNPs behaved differently from other AgNPs because it was a soluble system instead of a suspension system and the Ag was bonded to the polymer but not attached to matrixes. The aggregation of AgNPs on polystyrene was restricted to a confined area because of the existence of molecular entanglements in polystyrene. This would lead to a homogeneous AgNPs distribution in solution.
The synthesized PS-AgNPs was applied to the catalytic reaction of 4-nitrophenol. An undergraduate student, Kuan-Chung Chen, in Prof. Ching-Ping Liu’s lab conducted the catalytic reaction kinetic study independently under the guidance of Prof. Liu. Analysis of the kinetic study indicated that the catalytic reduction reaction of 4-nitrophenol was a pseudo-first order reaction by using excess NaBH4. The reaction rate constant showed a linear relationship to the concentrations of Ag in solution. The linear range of the rate constant of reduction reaction by using PS-AgNPs as catalyst was about 2-order range which outperformed other AgNPs (usually within 1-order range) for the same reaction. Additionally, the optimized rate constant using PS-AgNPs as catalyst was comparable to that of the highest record under the same Ag content.
Synergistic effect of the research effort of Prof. Ping-Tsung Huang’s lab (Organic Semiconducting Material Research Laboratory) and Prof. Ching-Ping Liu’s lab (Nanomaterials Research Lab) has completed an outstanding research work on applying a novel AgNPs material to the catalytic kinetic study of 4-nitrophenol. Financial support of this work from Ministry of Science and Technology (MOST) and Office of Research and Development of Fu Jen Catholic University are highly appreciated.
98 views