Featured Scientist

Ching-Ping Liu
College of Science and Engineering
Ching-Ping Liu
Department of Chemistry,
Fu Jen Catholic University,, New Taipei City, Taiwan
Article published in
"J. Mater. Chem. A", 2019,7, 20919-20925
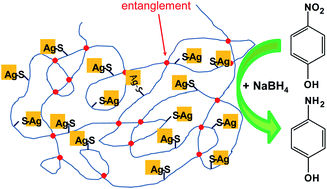
Confinement of silver nanoparticles in polystyrenes through molecular entanglements and their application for catalytic reduction of 4-nitrophenol
The limited stability and aggregation of colloidal nanoparticles are typically major issues in catalysis. In this work, a highly efficient polystyrene based nano-silver containing polymer (PS-AgNPs) has been synthesized as a catalyst for the reduction of 4-nitrophenol. Entanglements of polystyrene chains restrict the aggregation of AgNPs in the polymer side chains and give rise to a stable nano-silver domain size. The domain size of nano-silver in both low Ag content PS-AgNPs (L-PS-AgNPs) and high Ag content PS-AgNPs (H-PS-AgNPs) is around 15 nm (in films). No intensified Ag aggregation occurs as the Ag concentration increases. Based on kinetic measurements of the catalytic model reaction of 4-nitrophenol, the linear relationship between the rate constant kapp and different Ag concentrations in L-PS-AgNPs was extended by two orders of magnitude of the Ag concentration for the first time. These results indicated that AgNPs in L-PS-AgNPs were homogeneously distributed and exhibited excellent dispersion stability. Additionally, the catalytic performance of H-PS-AgNPs was comparable to a microreactor including the core of AgNPs/SiO2 and polymer shell. In this work, H-PS-AgNPs based on molecular entanglements were relatively simple as compared to the core/shell design of microreactors, which provided a new strategy to confine the size domain of metal nanoparticles immobilized on polymers.[Full article]